Abstract
Background: A gain in the number of copies of chromosome 1q (+1q) is one of the most common cytogenetic abnormalities (CA) detected in multiple myeloma (MM), however its role as a prognostic marker remains controversial. Here we evaluated clinical outcomes in patients with newly diagnosed MM patients with +1q abnormalities.
Methods: Patients with newly diagnosed MM seen at Princess Margaret Cancer Center (PMCC) between January 1, 2017 and March 31, 2021 who had cytogenetic testing for +1q were included in the study. Patients were excluded if they were only evaluated for a second opinion, if there was inadequate information to determine disease characteristics or treatment response, or if there was concurrent AL amyloidosis at diagnosis. Disease characteristics and treatment response data were collected from chart review.
Results: 284 patients were included in this study, of which 155 patients (54.6%) had +1q abnormalities. Median follow-up time was 20 months (IQR 12 - 32 months). +1q patients were older (median age 65 vs 63 p = 0.042), and predominantly female (56.1% vs 41.1%, p = 0.016). At presentation +1q patients had a lower hemoglobin (99.8 g/L vs 106.9 g/L, p = 0.009), higher proportion of elevated LDH (45.1% vs 29.8%, p = 0.015), higher beta-2 microglobulin (6.3 mg/L vs 5.3 mg/L, p = 0.04), and a trend towards higher creatinine (173.4 µmol/L vs 122.8 µmol/L, p = 0.053) compared to patients without +1q. A higher proportion of +1q patients had other high-risk CAs (34.8% vs 12.5%, p < 0.001). More +1q patients had R-ISS stage 2 and 3 disease compared to patients without +1q (p < 0.001).
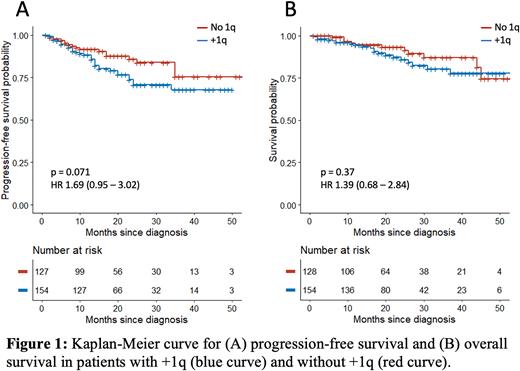
Most patients received proteasome inhibitor-based therapy (i.e, cyclophosphamide-bortezomib-dexamethasone) for first-line induction (85.2% in +1q, 93.5% without +1q, p = 0.088)). There was no difference in overall response rate (ORR) to induction therapy in +1q patients (87.3% vs 94.3%, p = 0.087) compared to those without +1q. A lower proportion of +1q patients were offered autologous stem cell transplant (ASCT, 72.3% vs 84.5%, p = 0.02). +1q patients who underwent ASCT had lower ORR compared to patients without +1q (92.1% vs 100%, p = 0.03). There was no significant difference in progression-free survival (PFS) in +1q patients compared to patients without +1q (HR 1.69, p = 0.074). Patients with other high-risk CAs who had +1q had lower PFS compared to those without +1q (p = 0.034). There was no difference in OS between +1q patients and those without +1q (HR 1.39, p = 0.37).
To evaluate the impact of +1q copy number, we compared patients with 3 copies of 1q (Gain1q) to ≥4 copies of 1q (Amp1q). There was no difference in baseline disease characteristics, response to first-line therapy or ASCT, PFS or OS between patients with Gain1q and Amp1q.
Conclusions: Over half of the patients investigated in this study had +1q abnormalities. +1q patients present at an older age, with more advanced disease. These patients had a lower ORR with ASCT; however, this did not impact their PFS or OS. Overall, +1q does not appear to be an independent marker of poor prognosis. Patients with other high-risk CAs with +1q may be considered an "ultra-high-risk". Our findings are in contrast to previously published retrospective analyses from the Mayo Clinic, MRC, and CoMMpass database1-3. Our study highlights the need for further prospective studies to better understand the prognostic significance of +1q abnormalities in MM.
References: 1. Abdallah N, Greipp P, Kapoor P, et al. Clinical characteristics and treatment outcomes of newly diagnosed multiple myeloma with chromosome 1q abnormalities. Blood Adv. 2020;4(11):3509-3519.
2. Schmidt TM, Barwick BG, Joseph N, et al. Gain of Chromosome 1q is associated with early progression in multiple myeloma patients treated with lenalidomide, bortezomib, and dexamethasone. Blood Cancer J. 2019;9(12):94.
3. Shah V, Sherborne AL, Walker BA, et al. Prediction of outcome in newly diagnosed myeloma: a meta-analysis of the molecular profiles of 1905 trial patients. Leukemia. 2018;32(1):102-110.
Disclosures
Smith:Bionano Genomics: Other: Personal Financial interest; Pfizer Canada: Membership on an entity's Board of Directors or advisory committees. Chen:Beigene: Consultancy; Abbvie: Consultancy; Novartis: Consultancy; BMS: Consultancy, Honoraria; Gilead: Consultancy, Honoraria, Research Funding. Prica:Kite-Gilead: Honoraria; Astra-Zeneca: Honoraria. Reece:Karyopharm: Consultancy; Amgen: Consultancy, Honoraria; Millenium: Research Funding; BMS: Research Funding; Merck: Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Otsuka: Research Funding; Sanofi: Honoraria; GSK: Honoraria. Stewart:Amgen: Consultancy; Janssen: Consultancy; Tempus Health: Consultancy, Other: Stock ownership (not including stocks owned in a managed portfolio; Sanofi: Consultancy; GlaxoSmithKline: Consultancy. Trudel:Genentech: Research Funding; BMS: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; GlaxoSmithKline: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; Sanofi: Honoraria; Pfizer: Honoraria, Research Funding; Forus: Consultancy; Janssen: Honoraria, Research Funding; AstraZeneca: Honoraria; Karyopharm: Honoraria; Takeda: Honoraria. Kukreti:Kwoya Kirin: Honoraria; EUSA Pharma: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal